News
Research team led by Professor Lyu Aiping and Professor Zhang Ge identifies new therapeutic target for osteoarthritis
30 April 2021
Osteoarthritis (OA) is the most common ageing-related degenerative joint disease affecting millions of people worldwide. Patients with OA suffer from continuous pain, and even deformity and disability of the affected joint. The pathological features of OA include ongoing degeneration of the articular cartilage as well as aberrant subchondral bone remodelling with hyperostosis of the joint. Unfortunately, there are no disease-modifying therapeutics known to effectively prevent or delay OA progression.
Research led by Professor Lyu Aiping, Dean of Chinese Medicine and Dr. Kennedy Y.H. Wong Endowed Professor in Chinese Medicine, and Professor Zhang Ge, Associate Dean (Research) of Chinese Medicine, found that the transfer of subchondral bone osteoclast-derived exosomal miRNA to chondrocytes reduced the resistance of cartilage to matrix degeneration, osteochondral angiogenesis and sensory innervation during OA progression. The results of this study have been published in the international scientific journal Nature Aging (https://www.nature.com/articles/s43587-021-00050-6).
To the surprise of the team, reduction in the resistance of cartilage to matrix degeneration, osteochondral angiogenesis and sensory innervation during OA progression was markedly retarded when the expression of Rab27a, a key intracellular molecule for regulating exosome secretion, was knocked down in the osteoclasts of OA mouse models. This finding prompted the team to further evaluate the potential therapeutic strategy of targeting the ostoeclastic exosome secretion to tackle OA.
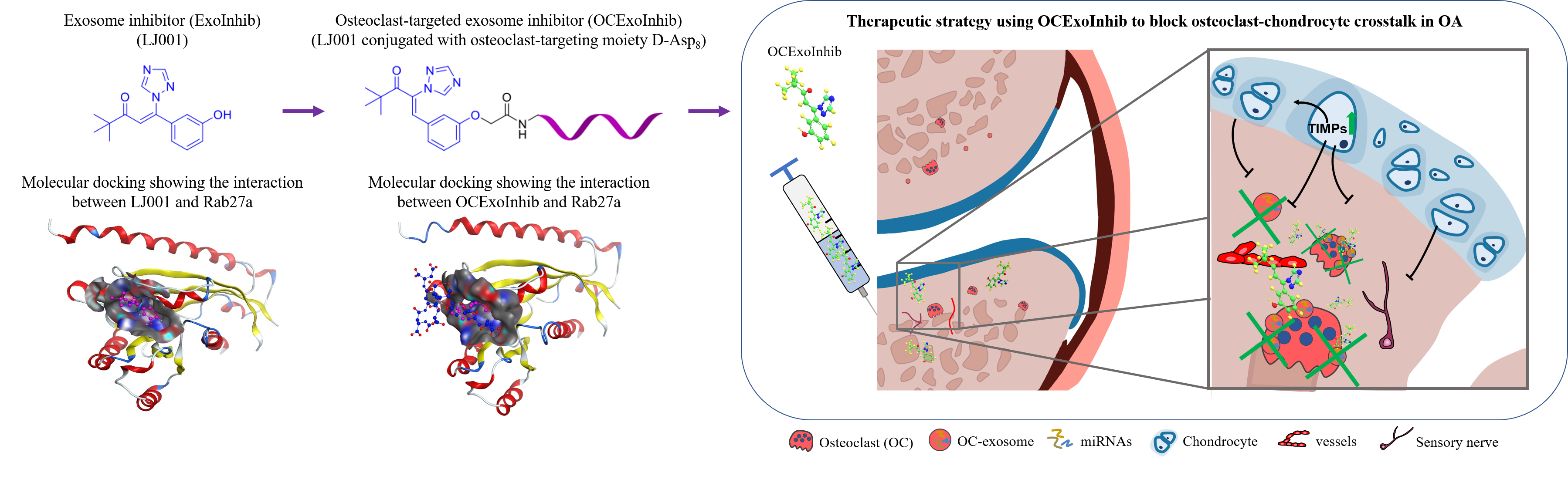
To facilitate the development of novel small molecule inhibitors targeting exosome secretion, the team modified the chemical structure of a previously reported Rab27a inhibitor, which could specifically block the Rab27a-dependent exocytosis but showed high cytotoxicity, by substituting the toxic nitro group with various other functional groups using molecular docking techniques. Among the new compounds so obtained, the team further identified LJ001 as a potent inhibitor candidate with prominent exosome inhibitory efficacy in vitro.
Dr. Liu Jin, Assistant Professor of the Teaching and Research Division (CMTR) and a key member of the team said: “From the translational aspect, it is important that the therapeutic administration of exosome inhibitor for OA treatment does not affect the exosome secretion from non-osteoclasts. It is because exosomes, as the emerging intracellular signals mediating cell-cell communication, are essential for physical function.” In view of this, the team designed a smart osteoclast-targeted exosome inhibitor by conjugating LJ001 with the osteoclast-targeting oligopeptide aptamer Asp8, which they developed previously. This novel osteoclast-targeted exosome inhibitor, i.e. Asp8-LJ001, was then synthesised using the well-established aptamer-drug conjugation technique in the lab. The team found that the OA progression was notably blunted in OA mouse model treated with Asp8-LJ001. Taken all together, the study has presented a promising strategy for innovative drug development for OA.
Other researchers in the team include Mr. Wu Xiaohao and Dr. Dang Lei, Research Assistants of CMTR; doctoral students Mr. Zhang Huarui and Mr. Zhong Chuanxin; and Professor Lu Jun from the School of Pharmacy at Chengdu University of Traditional Chinese Medicine and Dr. Huang Guangxin from Department of Joint Surgery at The Third Affiliated Hospital of Southern Medical University.